The periodic table is a familiar sight in classrooms and laboratories around the world. It’s a colorful grid that neatly organizes the elements, those fundamental building blocks of all matter. Every element, from the lightest hydrogen to the heaviest uranium, occupies its unique place in this table. But how much do we really know about these elements that make up everything we see and touch?

Image: english.ocr.org.uk
In this exploration, we’ll delve into a fascinating selection of 30 elements from the periodic table. We’ll uncover their intriguing properties, explore their diverse applications, and uncover how they shape our world in ways we often take for granted. Let’s embark on a journey through the captivating world of chemistry, one element at a time.
30 Key Players in the Periodic Table
The periodic table is a magnificent tapestry of elements, each with its own unique story to tell. To truly appreciate the beauty and complexity of this table, we must focus on individual elements. Within the vast array of 118 elements, we’ve chosen 30 key players that encompass a diverse range of properties and applications.
These 30 elements are not simply random selections; they represent the core elements that have shaped human civilization and continue to drive scientific innovation. Some are common and familiar, forming the very essence of our planet, while others are rare and precious, holding the key to technological advancements. Let’s start our journey of discovery with the first ten elements, those that make up the very foundation of our universe:
Elements 1-10: The Building Blocks of Creation
- Hydrogen (H): The lightest and most abundant element in the universe, Hydrogen fuels stars and is a critical component of water.
- Helium (He): Light and inert, Helium is used in balloons, as a coolant for magnets, and in the study of the cosmos.
- Lithium (Li): A soft, reactive metal used in batteries and as a mood stabilizer in medicine.
- Beryllium (Be): A strong, lightweight metal used in alloys and nuclear reactors.
- Boron (B): A metalloid used in insecticides, detergents, and as a component of borosilicate glass.
- Carbon (C): The backbone of life, Carbon forms the basis of all organic molecules and is used in countless materials and technologies.
- Nitrogen (N): The most abundant gas in the atmosphere, Nitrogen is vital for plant growth and is used in fertilizers and explosives.
- Oxygen (O): Essential for respiration, Oxygen also makes up water and is crucial for combustion.
- Fluorine (F): A highly reactive halogen used in dental care, non-stick cookware, and refrigerants.
- Neon (Ne): An inert gas that glows brightly when energized, used in signage and lasers.
Moving beyond the first ten, we encounter elements that showcase the wide spectrum of properties and uses. From the metals that shape our industries to the non-metals that power our electronics, these elements are essential components of our modern world:
Essential Elements for Modern Life
- Sodium (Na): Highly reactive alkali metal used in table salt, streetlights, and batteries.
- Magnesium (Mg): A lightweight and strong metal used in alloys, batteries, and in chlorophyll for photosynthesis.
- Aluminum (Al): The most abundant metal in the Earth’s crust, Aluminum is used in aircraft, packaging, and countless everyday items.
- Silicon (Si): The foundation of the semiconductor industry, Silicon is used in computers, smartphones, and solar panels.
- Phosphorus (P): A crucial component of DNA and ATP, Phosphorus is also used in fertilizers and matches.
- Sulfur (S): Used in sulfuric acid, a key industrial chemical, Sulfur is also found in fertilizers and rubber.
- Chlorine (Cl): A highly reactive halogen used in disinfectants, bleach, and PVC.
- Argon (Ar): An inert gas used in welding, lasers, and as a protective atmosphere.
- Potassium (K): An essential nutrient for plants and animals, Potassium is also used in fertilizers and batteries.
- Calcium (Ca): A vital component of bones and teeth, Calcium is also used in construction and cement.
The periodic table holds a wealth of fascinating elements, each with its own unique story. Continuing our exploration, we encounter elements that are rarer, more valuable, and often with specialized applications:
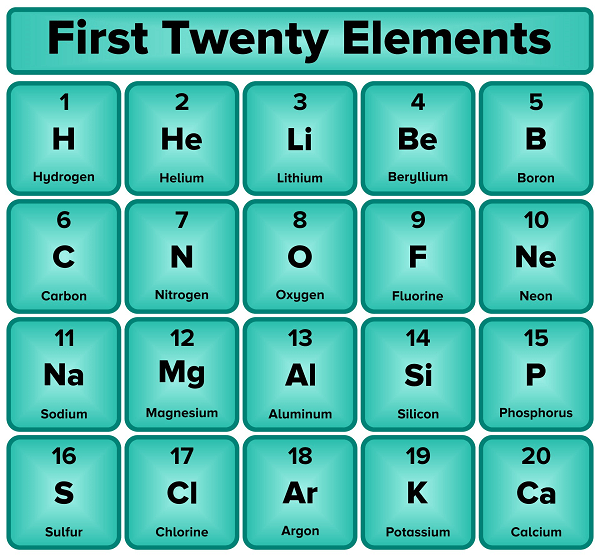
Image: www.javatpoint.com
Rarities and Specialties in the Periodic Table
- Scandium (Sc): A rare metal used in high-intensity lighting and as a component in alloys.
- Titanium (Ti): A strong, corrosion-resistant metal used in aerospace, implants, and jewelry.
- Vanadium (V): A strong metal used in alloys for tools and machinery.
- Chromium (Cr): Highly resistant to corrosion, Chromium is used in plating, stainless steel, and pigments.
- Manganese (Mn): Essential for plant growth, Manganese is also used in steel production.
- Iron (Fe): The most abundant metal on Earth, Iron is used in construction, machinery, and transportation.
- Cobalt (Co): Used in magnets, alloys, and as a catalyst.
- Nickel (Ni): A corrosion-resistant metal used in coins, batteries, and alloys.
- Copper (Cu): A highly conductive metal used in wiring, plumbing, and coins.
- Zinc (Zn): An essential mineral for health, Zinc is also used in batteries, alloys, and as a corrosion inhibitor.
The periodic table holds a treasure trove of scientific knowledge, and these 30 elements are just a small glimpse into its vastness. As we delve deeper into the properties and applications of each element, we gain a greater appreciation for the interconnectedness of matter and the remarkable progress that has been achieved through understanding the elements.
Tips for Exploring the Periodic Table Further
Exploring the periodic table can be a fascinating journey for students, researchers, and anyone who is curious about the building blocks of our world. Here are some tips for further exploration:
- Start with the basics: Begin by familiarizing yourself with the fundamental organization of the periodic table. Understand the meaning of groups and periods, and identify trends in atomic size, electronegativity, and ionization energy.
- Focus on specific groups: Explore the properties of elements within a particular group, such as the alkali metals, halogens, or noble gases. This will help you understand how elements behave within a family.
- Learn about applications: Investigate the real-world applications of elements. How are they used in our daily lives, in technologies, and in industries?
- Read about historical discoveries: Discover the fascinating stories behind the discovery of certain elements. Who were the pioneers who first isolated and characterized them?
- Connect with the scientific community: Engage with online resources, scientific blogs, and forums to stay informed about the latest developments in element research and applications.
By actively engaging with the periodic table, you can unlock a world of scientific wonders and gain a deeper understanding of the world around you. Each element has a unique story to tell, and together they weave a rich tapestry of matter and human ingenuity.
FAQ About the Periodic Table
What is the periodic table?
The periodic table is a tabular arrangement of the chemical elements, organized by their atomic number (number of protons), electron configuration, and recurring chemical properties. It is a fundamental tool in chemistry, providing a systematic framework for understanding the behavior and properties of elements.
How is the periodic table organized?
The periodic table is organized into rows (periods) and columns (groups). Elements in the same period have the same number of electron shells, while elements in the same group share similar chemical properties due to having the same number of valence electrons (electrons in the outermost shell).
What are some of the key trends in the periodic table?
Some key trends include:
- Atomic radius: Atomic radius generally increases down a group and decreases across a period.
- Electronegativity: Electronegativity generally increases across a period and decreases down a group.
- Ionization energy: Ionization energy generally increases across a period and decreases down a group.
What are some of the applications of elements?
Elements are used in a vast array of applications, from everyday materials to cutting-edge technologies. Some examples include:
- Hydrogen (H): Used as fuel and in the production of ammonia.
- Carbon (C): The backbone of organic molecules and used in numerous materials and technologies.
- Silicon (Si): The foundation of semiconductors and used in computers and solar panels.
- Iron (Fe): Used in construction, machinery, and transportation.
- Gold (Au): Used in jewelry, electronics, and dentistry.
What are some of the notable discoveries in the periodic table?
The discovery of the periodic table is a testament to human curiosity and scientific ingenuity. Some notable discoveries include:
- Dmitri Mendeleev: The Russian chemist who formulated the first version of the periodic table in 1869.
- Henry Moseley: The British physicist who refined the periodic table based on atomic numbers in 1913.
- Glenn Seaborg: The American chemist who synthesized and discovered several transuranic elements.
30 Elements Of The Periodic Table
https://youtube.com/watch?v=1qrLhx25dgk
Conclusion
The periodic table is a remarkable testament to the order and beauty found in the universe. These 30 elements represent just a small fraction of the fascinating atoms that make up our world. By understanding each element’s unique properties and applications, we gain a profound appreciation for the interconnectedness of nature and the vast potential that resides within the building blocks of matter. Are you curious to learn more about the elements of the periodic table? What elements are you most interested in learning more about?






