Remember those chemistry classes in high school? I certainly do! The concept of ions always seemed a bit daunting at first. Yet, as I delved deeper, I realized how essential understanding anions and cations is to grasp the fundamental building blocks of chemical bonding. It’s like learning the alphabet before you can read a book about chemistry. Today, I want to share with you a simple yet powerful resource: a list of anions and cations PDF. This is your handy guide to deciphering the world of chemical compounds and their interactions.
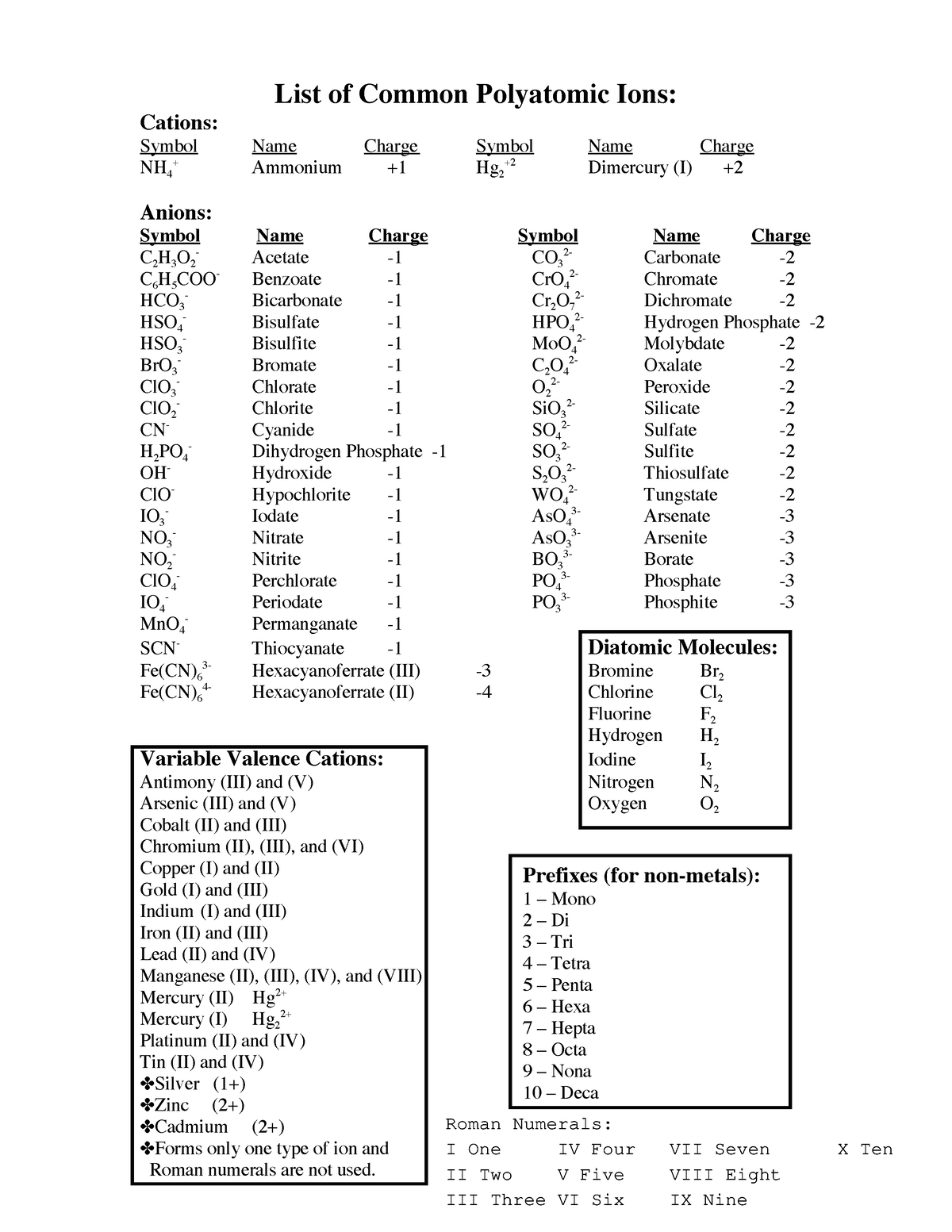
Image: www.studocu.com
Whether you’re a student, an aspiring chemist, or just someone who’s curious about the world around you, this list can be a valuable asset. But before diving into the benefits of having such a list, let’s understand what exactly anions and cations are and why they’re so important.
Understanding Anions and Cations
Atoms are the smallest units of an element that retain the chemical properties of that element. These atoms consist of a nucleus containing protons (positively charged) and neutrons (no charge) surrounded by electrons (negatively charged). Now, when an atom loses or gains electrons, it becomes an ion. This is where our two main players come into the picture: anions and cations.
Anions are negatively charged ions. They are formed when an atom gains one or more electrons. These extra electrons create a negative charge on the atom. For example, a chlorine atom (Cl) can gain an electron to become a chloride ion (Cl-), which is an anion.
Cations, on the other hand, are positively charged ions. They are formed when an atom loses one or more electrons. The loss of electrons leaves the atom with a net positive charge. For instance, a sodium atom (Na) can lose an electron to become a sodium ion (Na+), which is a cation.
The Importance of Ions
The concept of ions is crucial in chemistry because they form the basis of ionic bonding. This type of bond occurs when a cation and anion are attracted to each other due to their opposite charges. This electrostatic attraction holds the ions together, forming ionic compounds.
Many compounds we encounter in our daily lives, from table salt (NaCl) to minerals in our soil, are ionic compounds. Understanding the interactions between anions and cations helps us comprehend how these compounds form and why they possess certain properties.
The Benefits of a List of Anions and Cations PDF
Now, having a handy list of anions and cations in a PDF format can significantly simplify your learning and understanding of chemistry. Here are some of the benefits:
- Easily Referenced: Having a PDF means you can readily access the list anytime, anywhere, without needing internet connection.
- Organized and Clear Information: A well-structured PDF will present the information in an organized manner, making it easy to find the specific anion or cation you’re looking for.
- Comprehensive Coverage: The list should include a wide range of common anions and cations, encompassing both simple and complex ions.
- Print and Go: The PDF can be printed and used as a reference sheet during your studies or while tackling chemistry problems.
Imagine looking for the charge of a specific ion while solving a chemistry problem. With a PDF list always within reach, you can quickly find the information and continue working efficiently. This resource is particularly valuable for students preparing for exams or standardized tests.

Image: www.scribd.com
Finding the Right Anions and Cations PDF
You’ll find many online resources that offer free printable lists of anions and cations, but not all of them are created equal. Look for one that satisfies the following criteria:
- Comprehensive: Make sure the list includes a wide range of commonly encountered anions and cations.
- Organized: The list should be presented in a clear and logical order, making it easy to navigate and find the information you need.
- Printable: Choose a list that is printable for easy access and reference.
- Accurate: Double-check the accuracy of the information on the list before relying on it.
Expert Tips for Learning Anions and Cations
Learning about anions and cations can be a bit overwhelming at first. Here are some expert tips to make mastering this concept easier:
- Focus on Common Ions: Start with the most common anions and cations. This will build a strong foundation for future learning.
- Create Flashcards: Use flashcards to memorize the names, symbols, and charges of the ions. It’s a simple and effective method.
- Practice Writing Formulas: Once you’re comfortable with the ions, practice writing the formulas of ionic compounds. This reinforces your understanding of the charges and bonding.
- Visualize the Charges: Visualizing the charges on ions can help you better understand how they interact to form ionic compounds.
Remember, consistent practice is key to mastery. The more you use the information from your list and apply the tips above, the better you’ll grasp the concepts of anions and cations, paving the way for a deeper understanding of chemistry as a whole.
FAQs about Anions and Cations
Here’s a quick overview of some frequently asked questions about anions and cations:
Q1: Can ions have more than one charge?
A: Absolutely! Some elements can form multiple ions with different charges. For example, iron can form both Fe2+ and Fe3+ ions.
Q2: Why are some elements more likely to form anions than cations?
A: Electronegativity, the tendency of an atom to attract electrons, plays a major role. Elements with higher electronegativity tend to gain electrons and form anions. For example, nonmetals like chlorine have high electronegativity, so they readily form anions like chloride ions.
Q3: How can I tell if an element will form an anion or a cation?
A: There are general rules you can use: metals tend to lose electrons and form cations, while nonmetals tend to gain electrons and form anions. However, there are exceptions to these rules, so it’s best to consult a periodic table or a reliable list of ions for specific information.
List Of Anions And Cations Pdf
Conclusion: Exploring the Building Blocks of Chemistry
Understanding the fundamental concepts of anions and cations is essential for comprehending chemical bonding, reactions, and the properties of countless substances in our world. A comprehensive list of anions and cations in PDF format can serve as your reliable guide, helping you learn and remember these critical building blocks of chemistry. Now, are you interested in learning more about chemical bonding and exploring the fascinating world of ions? Let’s dive deeper together and uncover the secrets of chemical interactions!






