Ever wondered what happens when you drop a shiny piece of zinc into a blue copper sulfate solution? Or are you curious to know how the silver coating on your jewelry magically appears? The answer lies in a fascinating chemical dance called a single replacement reaction.
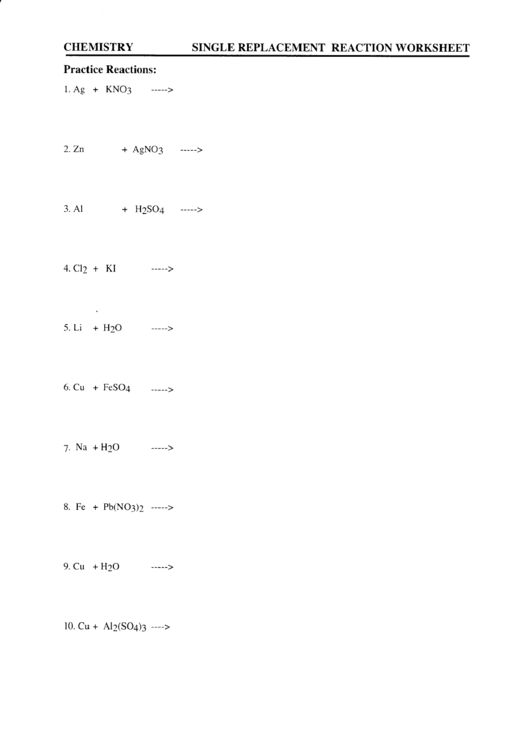
Image: printableella99.z21.web.core.windows.net
These reactions, also known as displacement reactions, are fundamental to our understanding of chemical transformations. They occur when a more reactive element replaces another in a compound. This process is a cornerstone of chemistry, and understanding it unlocks a deeper understanding of how elements interact and form new substances.
What is a Single Replacement Reaction, Anyway?
Imagine a game of musical chairs where elements are the players and chemical compounds are the chairs. In a single replacement reaction, a lone element, the “new player,” arrives and tries to claim a spot on a compound. If this new player is more reactive than the element currently occupying the spot, a swap happens. The more reactive element kicks out the less reactive one, forming a new compound and leaving the displaced element behind.
The Players: Elements and Compounds
In this chemical drama, we have two main players: the element and the compound. The element, the “new player,” is always in its elemental form, like zinc (Zn) or copper (Cu). The compound, the “chair,” is often made up of a metal and a nonmetal. For instance, copper sulfate (CuSO4) or silver nitrate (AgNO3).
The Rules: Reactivity Series
But not all elements are equal in their eagerness to displace other elements. That’s where the reactivity series comes in. This chart ranks elements based on their ability to lose electrons and form positive ions. Elements higher on the series are more reactive and will displace elements lower on the list.

Image: comedy1101.blogspot.com
The Outcome: The Exchange
If a more reactive element encounters a compound containing a less reactive element, a single replacement reaction occurs. Imagine placing a piece of zinc (Zn) into a solution of copper sulfate (CuSO4). Zinc (Zn) is higher on the reactivity series than copper (Cu). In this case, zinc will displace copper, resulting in the formation of zinc sulfate (ZnSO4) and elemental copper (Cu). The reaction looks like this:
Zn + CuSO4 → ZnSO4 + Cu
In essence, the zinc atom (Zn) loses electrons and becomes a positive zinc ion (Zn2+), while the copper ion (Cu2+) gains electrons and becomes neutral copper (Cu) metal. The zinc ion combines with the sulfate ion (SO42-) to form zinc sulfate.
Single Replacement Reaction Worksheet Answer Key: Cracking the Code
Navigating through a single replacement reaction worksheet can be a fun journey into the world of chemical reactions. But sometimes, those answers can be tricky to decipher. Let’s unravel the mysteries and decode some common types of problems found on these worksheets.
Predicting Products: The “Will it React?” Question
Many worksheets challenge you to predict the products of a potential single replacement reaction. To ace these questions, remember the golden rule: “Will the new element displace the element in the compound?” To answer, consult the reactivity series. Compare the reactivity of the “new player” element with the element bound in the compound. If the new element is more reactive, a reaction will occur, and you can predict the products.
Balancing Equations: The “Balancing Act”
Once you’ve identified the reactants and products, the next step is to balance the chemical equation. This ensures that the number of atoms of each element is equal on both sides of the equation, adhering to the law of conservation of mass.
Here’s a step-by-step approach:
- Write the unbalanced equation, representing the reactants and products.
- Count the number of atoms of each element on both sides of the equation.
- Adjust the coefficients (numbers in front of the chemical formulas) to balance the atoms of each element.
- Check if the number of atoms on both sides is the same.
For example, let’s balance the reaction between zinc and copper sulfate:
Zn + CuSO4 → ZnSO4 + Cu
In this equation, we have:
- 1 zinc atom (Zn) on the left and 1 zinc atom (Zn) on the right.
- 1 copper atom (Cu) on the left and 1 copper atom (Cu) on the right.
- 1 sulfur atom (S) on the left and 1 sulfur atom (S) on the right.
- 4 oxygen atoms (O) on the left and 4 oxygen atoms (O) on the right.
The equation is already balanced.
Identifying Reactants and Products: The “Who’s Who” Puzzle
Some worksheets present you with a scenario and ask you to identify the reactants and products. Analyze the description carefully. Look for clues like “a metal is added to a solution of a compound” or “a gas is released.” These clues will help you identify the participating elements and compounds.
Real-World Applications: Single Replacement Reactions in Action
Beyond the classroom, single replacement reactions are at work in our daily lives, influencing various processes and technologies.
1. Electroplating: Coating Metals with a Protective Layer
Electroplating involves using a single replacement reaction to coat a base metal with a thin layer of a more reactive metal. This process provides protection against corrosion, enhances conductivity, or adds a decorative finish.
Imagine a piece of jewelry being coated with a thin layer of silver. The silver ions in the solution are reduced and deposited onto the jewelry, replacing a less reactive metal like copper. Electroplating uses this principle to create various decorative and functional coatings on metallic surfaces.
2. Corrosion: The Degradation of Metals
Corrosion is a chemical process where metals react with their environment, often involving single replacement reactions. When iron (Fe) reacts with oxygen (O2) and water (H2O) in the presence of electrolytes, it undergoes a single replacement reaction to form iron(III) oxide (Fe2O3), commonly known as rust.
Rusting weakens the metal, leading to its degradation. This process underscores the importance of protective coatings and strategies to prevent corrosion in structures and machinery.
3. Metallurgy: Extracting Metals from Ores
Metallurgy, the process of extracting metals from their ores, often relies on single replacement reactions. In many cases, metals are combined with elements like oxygen to form oxides. To extract the metal, a more reactive element is used to displace the metal from its oxide, forming a new compound. For example, in the production of iron, carbon is used to displace iron from iron oxide ore.
The use of single replacement reactions in metallurgy underpins the production of essential metals like iron, copper, and aluminum, which play crucial roles in our modern world.
Single Replacement Reaction Worksheet Answer Key
Conclusion: Unleashing the Power of Single Replacement Reactions
Unraveling the intricate details of single replacement reactions, mastering the use of the reactivity series, and understanding the practical applications of this fundamental chemical process empowers us to delve deeper into the fascinating world of chemistry. These reactions are not merely abstract concepts confined to textbooks but rather powerful forces shaping our world, from the intricate coatings on our jewelry to the extraction of essential metals.
So, let’s embrace the challenge of single replacement reaction worksheets and unlock the secrets hidden within their answers. The journey of understanding these reactions takes us beyond the confines of chemical equations and unveils the intricate interplay of elements and compounds that govern our world.






