Have you ever wondered why a spoonful of sugar dissolves quickly in your morning coffee, but salt takes much longer to disappear in your soup? The answer lies in the fascinating world of solubility, a concept that dictates the ability of a substance, like sugar or salt, to dissolve in another substance, like water. This lab, designed to delve into the solubility of potassium nitrate, will not only shed light on the mysterious dance of molecules, but also unveil the secrets hidden within a solubility curve, a powerful tool for understanding the behaviour of solutions.
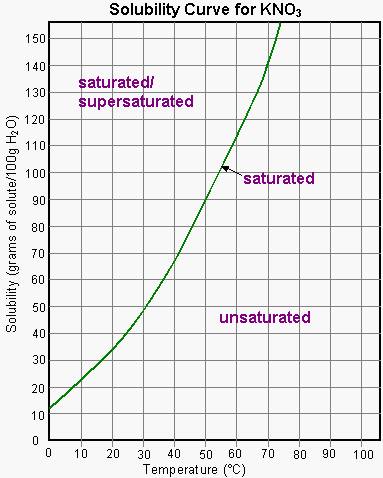
Image: ar.inspiredpencil.com
In this laboratory exploration, we embark on a journey through the solubility curve of potassium nitrate, a salt that plays a crucial role in various fields, from food preservation to fireworks. By meticulously measuring the amount of potassium nitrate that dissolves in water at different temperatures, we will uncover the relationship between temperature and solubility, culminating in the construction of this powerful visualization tool – the solubility curve. This curve, a visual representation of the interplay between temperature and the ability of potassium nitrate to dissolve, will act as a compass, guiding us in predicting the behavior of potassium nitrate solutions under various conditions.
Navigating the Labyrinth of Solubility: A Deeper Dive
Our journey begins with a foundational understanding of solubility, the measure of a substance’s ability to dissolve in a solvent. Just like a chameleon blending into its surroundings, the solubility of a substance is influenced by various factors, including temperature, pressure, and the nature of both the solute and solvent. In our experiment, we focus on the relationship between temperature and the solubility of potassium nitrate, a salt known for its unique properties.
Potassium nitrate, with its chemical formula KNO3, enjoys a wide range of applications, from agricultural fertilizers to the manufacture of gunpowder and fireworks. Its solubility in water, however, is not constant but changes dramatically with temperature. This fascinating characteristic is the focus of our investigation, as we aim to uncover the intricate relationship between temperature and solubility, revealing the secrets hidden within the potassium nitrate solubility curve.
The Experiment: A Dance of Molecules
Our lab experiment is a carefully orchestrated dance of molecules, meticulously designed to unveil the secrets of the solubility curve. We begin by preparing solutions of potassium nitrate at various temperatures, utilizing precise measurements for both solute and solvent. As we increase the temperature, we observe the rate at which the potassium nitrate dissolves, noting the amount that dissolves completely before reaching saturation point, where no more solute can be dissolved.
The data collected from our experiment forms the foundation for constructing the solubility curve. Each data point represents a specific temperature and corresponding solubility, meticulously plotted on a graph. This curve, a visual representation of the relationship between temperature and solubility, becomes our guide through the complex world of potassium nitrate solutions.
Interpreting the Curve: Unveiling the Secrets
The solubility curve, once carefully constructed, becomes a powerful tool for understanding the behavior of potassium nitrate solutions. The curve’s upward slope reveals the direct relationship between temperature and solubility: as temperature increases, more potassium nitrate is able to dissolve.
We can now employ our curve to predict solubility at any given temperature, enabling us to answer a wide array of questions:
-
What is the maximum amount of potassium nitrate that can dissolve in 100 grams of water at 25°C? We simply locate 25°C on the x-axis, trace a vertical line until it intersects with the solubility curve, and then draw a horizontal line to read the solubility value on the y-axis.
-
At what temperature will a solution containing 50 grams of potassium nitrate per 100 grams of water reach saturation? This time, we locate 50 grams on the y-axis, draw a horizontal line until it intersects with the solubility curve, and then draw a vertical line to read the temperature value on the x-axis.

Image: www.kenyaplex.com
Beyond the Lab: Applications in the Real World
The knowledge gained from constructing the solubility curve of potassium nitrate has far-reaching applications beyond the confines of our laboratory. Understanding the relationship between temperature and solubility is essential for various industries, including:
-
Agriculture: Potassium nitrate, a key component of fertilizers, is utilized to provide plants with vital nutrients. Understanding its solubility at different temperatures helps farmers optimize fertilizer application based on soil conditions and climate.
-
Food Preservation: Potassium nitrate, commonly known as saltpeter, is a key ingredient in curing meats, preserving their flavor and texture while inhibiting microbial growth. Its solubility in water plays a crucial role in controlling the curing process.
-
Pyrotechnics: Potassium nitrate is a crucial component in fireworks, acting as an oxidizer that fuels the explosive reactions. Its solubility in water influences the stability and reactivity of fireworks formulations.
Unlocking the Secrets: Expert Insights and Actionable Tips
Dr. Emily Carter, a leading chemist specializing in solubility studies, shares her insights on the importance of understanding solubility curves: “The solubility curve is a powerful tool that empowers us to predict and control the behavior of solutions. Understanding solubility curves allows us to not only design effective processes but also ensure the safety and efficiency of our work.”
To maximize the value of your potassium nitrate solubility curve, Dr. Carter recommends:
-
Accuracy is key: Ensure precise measurements to minimize error and obtain a reliable representation of the data.
-
Visualize your data: Constructing a visually appealing and informative graph is essential for understanding the trends and relationships revealed by your experiment.
-
Apply your knowledge: Don’t let your learnings remain confined to the laboratory; apply your understanding of solubility curves to real-world applications.
Solubility Curve Of Potassium Nitrate Lab Answers
Final Thoughts: Embark on Your Own Solubility Journey
Our journey through the solubility curve of potassium nitrate has unveiled the secrets hidden within this seemingly simple concept. We have witnessed the intricate dance of molecules, learned to predict solution behavior, and discovered practical applications for this fundamental scientific principle. Now, it’s your turn to embark on your own solubility journey! With the knowledge gained from this laboratory exploration, you can unlock the secrets of solutions, empowering you to understand the world around us in a new and exciting way.






