Have you ever wondered how scientists precisely measure the strength of an acid or base? Picture a chemist carefully dripping a solution into a beaker, monitoring the reaction with a watchful eye. That’s a titration, a crucial technique employed in chemistry and various industries. This article delves into the fascinating world of titrations, exploring its history, principles, and applications in the everyday world.
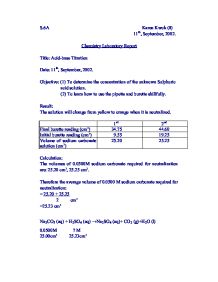
Image: petelevin.com
Imagine trying to bake a cake without accurately measuring the ingredients. The outcome would be unpredictable, possibly a disastrous culinary experience! Titration in chemistry is like the measuring cup for acids and bases, allowing us to determine their concentration with precision. It’s a cornerstone of analytical chemistry used in various fields, from environmental monitoring to pharmaceutical development.
Unveiling the Secrets of Titration: A Journey Through History
The roots of titration can be traced back to the 17th century, where French chemist Antoine Lavoisier experimented with the reaction between acids and bases. He observed that these reactions involve the formation of salts and water. However, it wasn’t until the 18th century that the concept of titration was formalized by French chemist François-Antoine-Henri Descroizilles.
Descroizilles introduced the first burette, a graduated tube used to measure the volume of titrant added to a solution. This invention revolutionized the way chemists determined the concentration of solutions. Over time, titrations evolved, incorporating various techniques and instruments, evolving from basic observation to sophisticated methods utilizing electronic sensors and computer analysis.
The Fundamentals of Titration: A Chemical Balancing Act
Titration is a quantitative analytical technique used to determine the concentration of a known solution, called the analyte, by reacting it with a solution of known concentration, called the titrant. The reaction between the analyte and titrant is a neutralization reaction, where the acid and base react to form salt and water.
The key to titration is the indicator, a substance that changes color depending on the solution’s pH. As the titrant is slowly added to the analyte, the pH of the solution changes. When the analyte and titrant reach the equivalence point, where the acid and base have neutralized each other, the indicator changes color, signaling the end of the titration.
Imagine adding drops of lemon juice to a mixture of baking soda and water. As the lemon juice (acid) neutralizes the baking soda (base), the mixture eventually stops fizzing. The indicator in this case is the fizz itself, a visual cue indicating the neutralization of the base.
A Closer Look at the Titration Process
The titration process typically involves the following steps:
- Preparation: Carefully prepare the analyte solution and titrant, ensuring their concentrations are accurately known.
- Titration: Add the titrant to the analyte, drop by drop, using a burette. This is crucial for precise volume control.
- Monitoring: Observe the color change of the indicator as the titrant is added.
- Equivalence Point: Record the volume of titrant added when the indicator changes color, signifying the equivalence point.
- Calculation: Use the volume of titrant and its known concentration to calculate the concentration of the analyte.
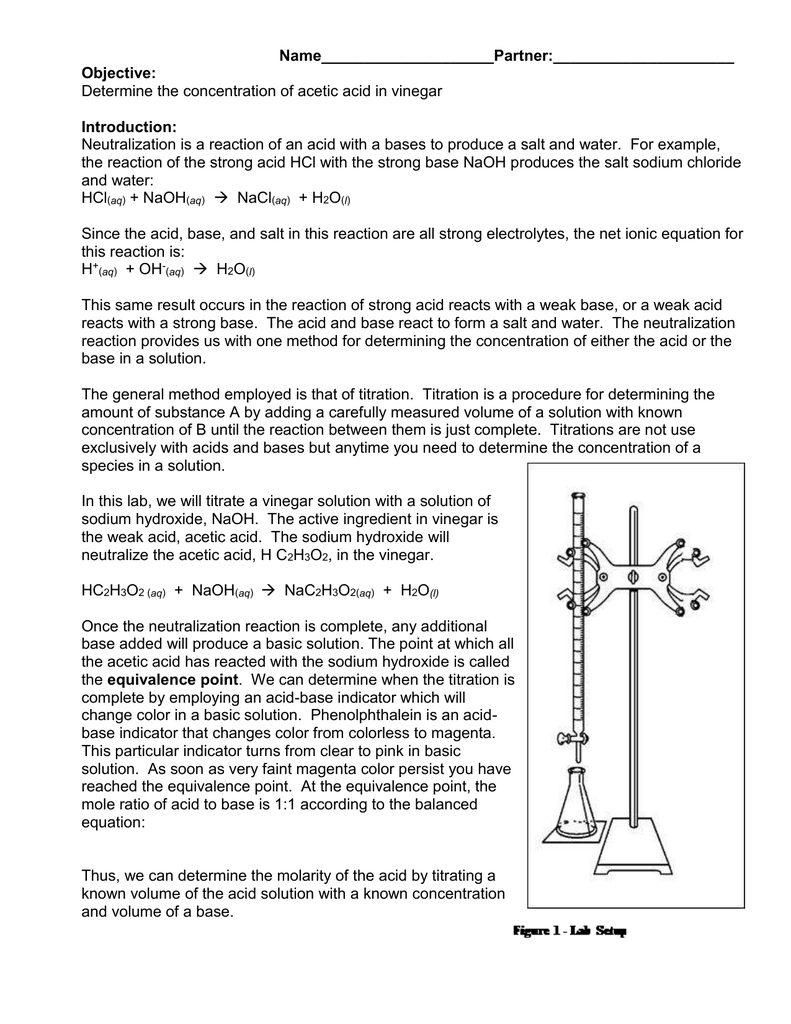
Image: mavink.com
Types of Titration: Navigating the Chemical Landscape
Titration techniques are not one-size-fits-all. Different chemistries demand specific approaches. Here are some common types of titrations:
Acid-Base Titration
This is the most common type, where a strong acid is used to titrate a base or vice-versa. It’s used to determine the concentration of unknown acids or bases.
Redox Titration
This involves a reaction between oxidizing and reducing agents to determine the concentration of an oxidizing or reducing analyte. It’s widely employed in environmental analysis to determine the concentration of pollutants.
Precipitation Titration
This type utilizes the formation of a precipitate to determine the analyte’s concentration. It’s commonly used to determine the concentration of chloride ions in water solutions.
Complexometric Titration
This involves the formation of a complex between the analyte and titrant, often used to determine the concentration of metal ions. It’s invaluable in analyzing the purity of metal samples.
Applications of Titration: A Multifaceted Tool
Titration’s precision has earned it a prominent role in various fields, including:
Environmental Monitoring
Titration helps determine the acidity or alkalinity of water samples, monitoring water quality to safeguard human health and ecosystems.
Pharmaceutical Industry
Titration ensures the purity and concentration of active ingredients in pharmaceuticals, guaranteeing their efficacy and safety.
Food and Beverage Industry
Titration plays a vital role in controlling the acidity of food products, ensuring their flavor and shelf life.
Industrial Chemistry
Titration is instrumental in analyzing raw materials, monitoring production processes, and ensuring the quality of finished products in numerous industries.
The Future of Titration: Advancements and Innovations
Titration is constantly evolving, embracing new technologies and techniques to improve accuracy, efficiency, and automation. Here are some exciting future directions:
Automated Titrators
These systems streamline the titration process, reducing human error and increasing efficiency. They incorporate sensors and automated data analysis, making titrations faster and more reliable.
Microfluidic Titration
This innovative technique uses microfluidic chips to perform titrations on minute volumes of samples, opening up new avenues for analyzing precious or limited samples.
Electrochemical Titration
This approach combines traditional titrations with electrochemical measurements, providing real-time information about the reaction’s progress. This offers significant advantages in terms of sensitivity and speed.
Titration Acid And Base Lab Report
A Final Word: The Enduring Relevance of Titration
Titration remains an invaluable tool for chemists, educators, and professionals across various industries. Its ability to determine the concentration of solutions with precision makes it a cornerstone of chemical analysis. From the laboratory to the production line, titration empowers us to understand the world around us with accuracy and efficiency.
As we continue to refine and innovate titration methods, its role in science, technology, and our daily lives will only grow. So, the next time you see a chemist carefully dripping a solution into a beaker, remember that it’s more than just a simple experiment; it’s a journey to unlock the secrets of acids and bases using one of the most versatile techniques in chemistry.






