Have you ever wondered why a feather floats while a rock sinks? Or why a balloon filled with helium can easily lift you off the ground? The answer lies in a fundamental concept in science: density. It’s about how much ‘stuff’ is packed into a given space. This concept, interconnected with volume and mass, plays a crucial role in understanding the world around us. And the best way to get an intimate grasp of these relationships is through hands-on practice, such as completing worksheets that explore the fascinating interplay of density, volume, and mass.
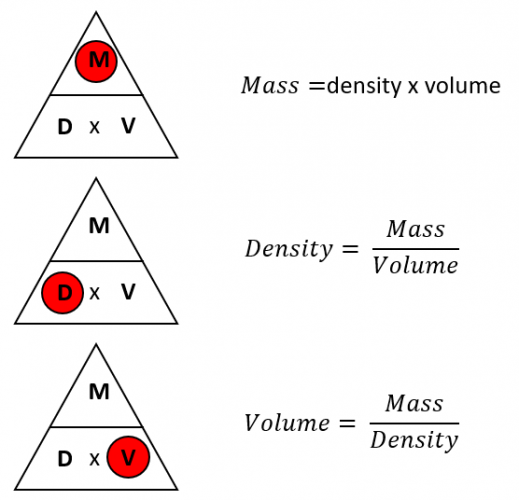
Image: www.tpsearchtool.com
Today, we’ll embark on a journey through these fundamental scientific concepts, unraveling their workings and mastering their applications. We’ll delve into the secrets of density, volume, and mass, armed with a worksheet filled with captivating problems. Together, we’ll explore how these concepts are intricately intertwined and how they explain the behaviors of objects in our world. Are you ready to dive in?
Understanding Density: The Heart of the Matter
Imagine you have two identical containers, one filled with feathers and the other with sand. Even though they occupy the same volume, they weigh very differently, right? This difference in weight is because of the different densities of the objects. Density, essentially, tells us how tightly packed the matter is in a given space. Think of it as a measure of ‘heaviness’ per unit volume.
To calculate density, we simply divide the mass of an object by its volume:
Density = Mass / Volume
Let’s break down these terms:
- Mass: The amount of matter in an object (often measured in grams or kilograms). It represents how much ‘stuff’ the object contains.
- Volume: The amount of space an object occupies (often measured in cubic centimeters or liters). It’s a measure of the object’s size.
- Density: A measure of how much mass is packed into a given volume. It is often measured in grams per cubic centimeter (g/cm3) or kilograms per cubic meter (kg/m3).
The Importance of Density
Density is more than just a scientific concept. It has far-reaching implications in our daily lives:
- Floating and Sinking: Objects less dense than water float, while those denser than water sink. This explains why ships made of steel can float (due to their hollow shape that increases their volume and decreases their overall density), while a rock, a denser object, sinks.
- Meteorology: Density plays a crucial role in understanding weather patterns. Warm air is less dense than cold air, causing it to rise, creating convection currents responsible for winds and storms.
- Material Science: Understanding density is crucial in material science. Engineers rely on it when choosing the right materials for various applications. For instance, building a light yet strong airplane requires a material with a high strength-to-density ratio.
Applying Density Concepts: Our Worksheet Adventure
Now, let’s put our knowledge to the test with a worksheet designed to solidify our understanding of density, volume, and mass. The worksheet features various scenarios and questions involving these concepts. You’ll have the opportunity to calculate density, determine the mass or volume of an object given its density and another parameter, and even explore practical applications like comparing the densities of different substances.

Image: studylib.net
Sample Worksheet Problems
Let’s look at a few sample problems from the worksheet to get a feel for its challenges:
Problem 1:
A block of wood has a volume of 100 cm3 and a mass of 80 grams. What is its density?
Problem 2:
A marble has a density of 2.5 g/cm3 and a volume of 5 cm3. What is its mass?
Problem 3:
You have two containers: one contains 1 liter of water, and the other contains 1 liter of oil. Which container has a higher density?
Unlocking the Answers
Don’t worry; you’re not alone in your worksheet journey. We’re here to provide you with answers to these intriguing problems:
Solution 1:
Density = Mass / Volume = 80 grams / 100 cm3 = 0.8 g/cm3
Solution 2:
Mass = Density * Volume = 2.5 g/cm3 * 5 cm3 = 12.5 grams
Solution 3:
Water has a higher density than oil. This means that water is denser, meaning it has more mass packed into a given volume.
Beyond the Worksheet: Exploring Density In-Depth
Completing the worksheet is a great way to solidify your understanding of these fundamental concepts. But the learning doesn’t stop there. Here are some real-world applications to explore further:
- Archimedes’ Principle: Explore how buoyancy is related to density. Archimedes’ Principle states that the buoyant force on an object submerged in a fluid is equal to the weight of the fluid that the object displaces.
- Density of Gases: Investigate how the density of gases affects their buoyancy and movement. Hot air balloons are a prime example of this!
- Density in Everyday Objects: Observe how density plays a role in the design and functionality of various objects in your daily life. Think about the design of a boat, the weight of a car, or the material used for a soda can.
The world is full of fascinating examples of density in action. As you continue to explore these concepts, you’ll gain a deeper appreciation for how density shapes the world around us.
Density Volume Mass Worksheet With Answers
Final Thoughts
Congratulations on completing our worksheet adventure! You’ve successfully honed your understanding of density, volume, and mass, revealing their crucial role in explaining the properties and behaviors of objects around us. As you venture further into the realm of science, remember that density is a key concept for unlocking a vast array of scientific mysteries. Keep exploring, keep questioning, and keep learning!






