Remember that daunting moment in chemistry class when your teacher introduced chemical equations? The world of reactants and products, coefficients and subscripts seemed like a foreign language. It wasn’t until we started working on balancing equations that I truly understood the elegant dance of atoms within chemical reactions. The concept of balancing, making sure the same number of atoms of each element appear on both sides of the equation, was a revelation. It wasn’t just about memorizing formulas; it was about understanding the fundamental law of conservation of mass – the universe simply doesn’t create or destroy matter. Balancing chemical equations ensures that this law remains inviolable.
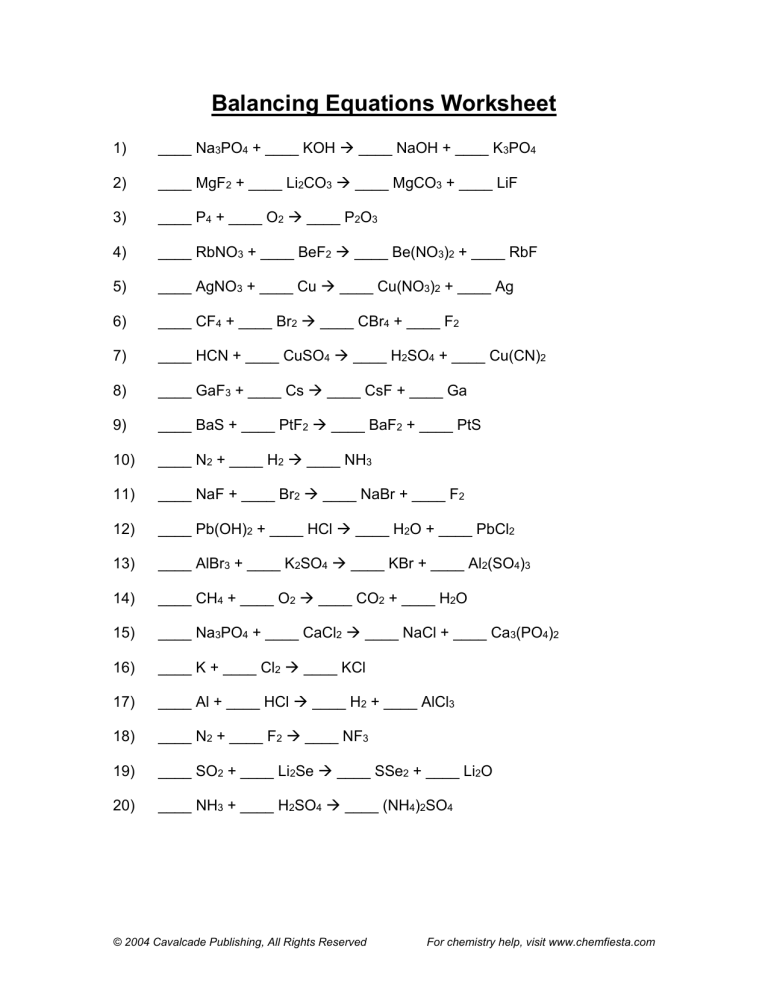
Image: classburns.z4.web.core.windows.net
Unit 7 Worksheet 2, often found in high school chemistry courses, takes this concept of balancing chemical equations to the next level. It typically introduces challenging scenarios with complicated reactions and multiple elements. This worksheet serves as a stepping stone towards a deeper understanding of stoichiometry, the art of quantifying and predicting chemical reactions. This blog post delves into the intricacies of Unit 7 Worksheet 2, explaining the key principles, unraveling common challenges, and offering tips to conquer this crucial step in your chemistry journey.
Decoding the Language of Chemical Reactions: Unraveling Balancing Techniques
At the heart of Unit 7 Worksheet 2 lies the understanding of chemical reactions and the importance of balancing them. Chemical reactions are simply the processes where substances, the reactants, transform into different substances, the products. Balancing these reactions ensures that the number of atoms of each element remains constant throughout the transformation. This principle, known as the law of conservation of mass, is fundamental to the very fabric of chemistry.
There are a few key techniques to master when balancing chemical reactions. The most common approach involves adjusting the coefficients, the numbers placed in front of the chemical formulas in an equation. By changing these coefficients, you can alter the number of molecules of each reactant and product, thereby adjusting the number of atoms on each side of the equation until they are equal. This process may seem daunting at first, but with practice, it becomes second nature.
Breaking Down the Balancing Process: Step-by-Step Guide
Let’s break down the process of balancing chemical reactions into a series of manageable steps. This systematic approach will help you tackle even the most complex reactions:
- Identify the Reactants and Products: Begin by carefully examining the chemical equation. Clearly identify the reactants, the substances you start with, and the products, the substances formed.
- Count the Atoms of Each Element: Create a table or list to track the number of atoms of each element on both the reactant and product sides of the equation.
- Balance One Element at a Time: Start with an element that appears only once on each side of the equation. Adjust the coefficients in front of the chemical formulas to make the number of atoms of that element equal on both sides.
- Repeat for Other Elements: Move on to another element and repeat the balancing process. Remember, you can only adjust coefficients, not subscripts (the small numbers in a chemical formula).
- Double-Check the Balanced Equation: Once you’ve balanced all elements, meticulously double-check your equation to ensure the number of atoms of each element is the same on both sides.
Overcoming Common Balancing Hurdles: Tips and Techniques
Balancing chemical equations can be tricky, especially when dealing with complex reactions. Here are a few helpful tips and techniques to overcome common hurdles:
- Polysatomic Ions: Sometimes, balancing reactions involves polyatomic ions, groups of atoms that act as a single unit. Treat these ions as single entities when balancing and adjust coefficients accordingly. For instance, if you have SO42- (sulfate ion) on both sides, balance them as a whole, not individually.
- Trial and Error: Balancing equations often involves a bit of trial and error. Don’t be discouraged if your first attempt doesn’t work. Experiment with different coefficients until you achieve balance.
- Fractional Coefficients: You may encounter fractional coefficients during the balancing process. If so, simply multiply the entire equation by the smallest common denominator to eliminate the fractions. For instance, if you have 1/2 H2O, multiply the entire equation by 2.
- Balance in Phases: If the reaction takes place in different phases, such as aqueous (aq) or gaseous (g), be sure to include these phase designations in your balanced equation.

Image: worksheetlistmg.z13.web.core.windows.net
Leveraging Online Resources and Tools: Enhancing Your Skills
The digital age offers a wealth of resources and tools to assist you in mastering chemical equation balancing. Many websites provide interactive balancing exercises, step-by-step explanations, and even virtual simulations that help you visualize the process. For instance, online balancing calculators allow you to input the unbalanced equation and instantly obtain the balanced version. These tools can be extremely helpful for reinforcing your understanding and practicing various scenarios. There are fantastic YouTube tutorials and online forums where you can connect with other students and seek clarification on specific challenges.
Real-World Relevance: The Importance of Balanced Equations
Balancing chemical equations is not just an academic exercise; it has real-world applications in various fields. In industrial processes, balancing equations helps ensure optimal product yields and minimize waste. In medicine, balanced equations are critical for understanding the reactions involved in drug development and the precise dosages of medications. Even in everyday life, we encounter balanced equations, such as the process of combustion in our cars or the reaction between baking soda and vinegar in a kitchen experiment.
Q&A: Addressing Common Queries About Balancing Chemical Reactions
What happens if a chemical equation is not balanced?
An unbalanced chemical equation violates the law of conservation of mass. It implies that atoms are being created or destroyed, which is impossible. Therefore, an unbalanced equation does not accurately represent the real chemical process.
Is there a shortcut to balancing chemical equations?
While there are tools like online calculators that can balance equations quickly, understanding the technique and practice are essential. These shortcuts can help, but they shouldn’t replace the fundamental understanding of the process.
Can I change the subscripts in a chemical formula to balance an equation?
No. Changing subscripts alters the chemical formula itself, effectively changing the chemical species involved in the reaction. This is incorrect and alters the identity of the substances involved.
Why is it important to balance chemical equations in stoichiometry?
Balancing equations in stoichiometry allows us to calculate the exact amount of reactants needed to produce a specific amount of product. This is crucial for accurate predictions and efficient chemical reactions in various applications.
Unit 7 Balancing Chemical Reactions Worksheet 2
Conclusion: Embracing the Dynamic World of Chemistry
Understanding and mastering the art of balancing chemical reactions is a cornerstone of a solid chemistry foundation. Unit 7 Worksheet 2, with its challenging scenarios, serves as a valuable stepping stone towards a deeper understanding of stoichiometry and the fundamental principles that govern chemical transformations. As you delve into the world of chemistry, remember that balancing equations is not just about numbers and symbols; it’s about understanding the fundamental dance of atoms and the elegant laws that govern our universe.
Are you ready to conquer the challenges of Unit 7 Worksheet 2 and embrace the dynamic world of chemistry? Share your thoughts and experiences in the comments below! We’re here to help you navigate this fascinating journey of chemical discovery.






