Have you ever wondered what holds atoms together to form the world around us? From the air we breathe to the water we drink, everything is composed of tiny particles called atoms, and these atoms are held together by invisible forces known as chemical bonds. Understanding these bonds is like unlocking a secret language that explains how molecules are formed and how matter interacts. In this article, we’ll explore the fascinating world of chemical bonding, focusing on two fundamental types: ionic and covalent bonds.
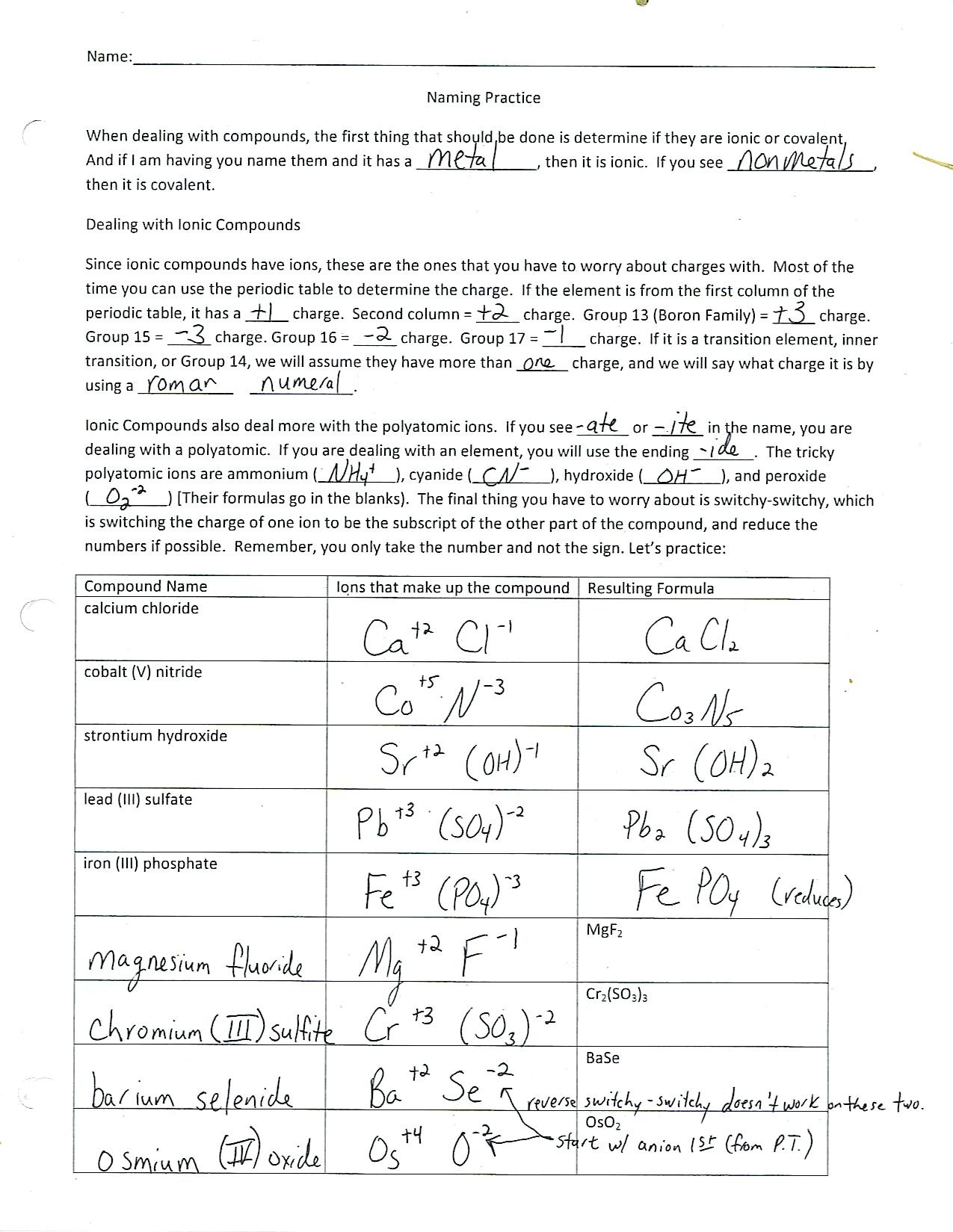
Image: worksheetlibrarycopp.z13.web.core.windows.net
This journey into chemical bonding starts with a little adventure – a worksheet. Worksheets, often perceived as monotonous schoolwork, can be surprisingly fun and informative when used creatively. By engaging with the concepts presented in the worksheet, you’ll start visualizing the invisible forces that bind atoms together, learn to predict the types of bonds that form, and gain a deeper appreciation for the building blocks of the universe.
The Foundation: Understanding Atoms
The Building Blocks of Matter
Before diving into the world of bonds, let’s quickly revisit the foundation. Everything around us is made of atoms, the smallest unit of an element that retains the chemical properties of that element. These tiny particles are like tiny solar systems, with a dense nucleus at the center and electrons orbiting around it. The nucleus consists of protons, positively charged particles, and neutrons, neutral particles. Electrons, negatively charged particles, whirl around the nucleus in specific energy levels called shells.
Chemical Bonding: The Force of Attraction
Atoms are constantly seeking stability. To achieve this, they form chemical bonds with other atoms, sharing or transferring electrons to gain a full outer shell of electrons. This stable configuration is often referred to as the “octet rule,” where atoms strive to have eight electrons in their outermost shell.
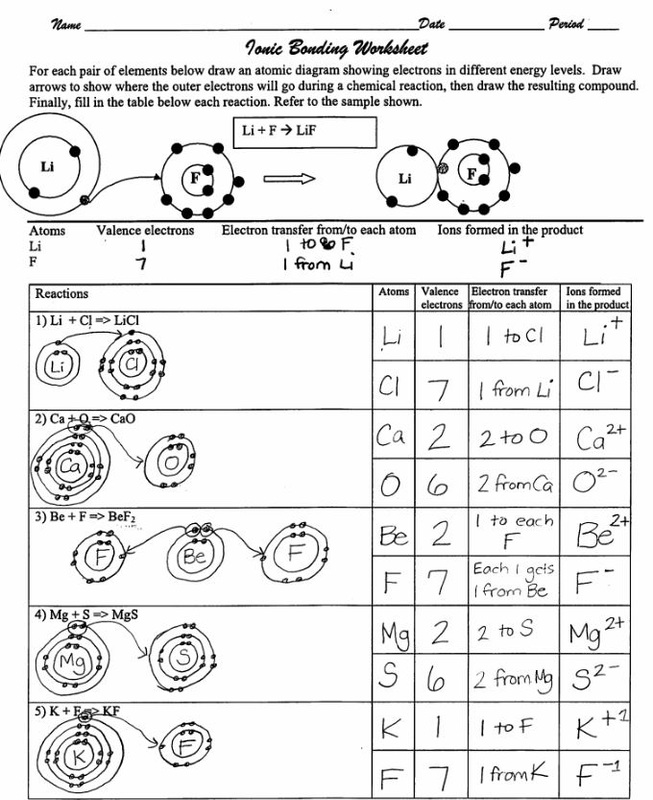
Image: www.myxxgirl.com
Exploring the Two Main Types of Bonds
Ionic Bonding: The Power of Transfer
Ionic bonding, like a good play, involves a transfer of electrons between atoms. In this process, one atom, usually a metal, loses electrons, becoming positively charged, and forms a cation. The other atom, typically a nonmetal, gains these electrons, becoming negatively charged and forming an anion. These opposite charges attract each other strongly, forming an ionic bond. This electrostatic attraction between oppositely charged ions is what holds the ions together in a crystal lattice structure.
Example: Consider the formation of sodium chloride (NaCl), commonly known as table salt. Sodium (Na) has one electron in its outer shell, while chlorine (Cl) has seven. Sodium readily loses its electron to achieve a stable configuration, forming a positively charged sodium ion (Na+). Chlorine gains this electron, becoming a negatively charged chloride ion (Cl-). The strong electrostatic attraction between these oppositely charged ions forms the ionic bond, resulting in the formation of table salt.
Covalent Bonding: Sharing is Caring
Covalent bonding is a bit more collaborative, with atoms sharing electrons to achieve stability. Instead of a complete transfer of electrons, atoms in a covalent bond share their outer-shell electrons to complete their octet. This sharing creates a stable molecule where both atoms have a complete outer shell. Covalent bonds can be formed between two atoms of the same element (like in O2, oxygen gas) or between two different elements (like in H2O, water).
Example: Let’s look at the formation of water (H2O). Each hydrogen atom has one electron in its outer shell. Oxygen, on the other hand, has six electrons in its outer shell. When two hydrogen atoms share their electrons with the oxygen atom, all three atoms achieve a stable configuration. This sharing of electrons results in two covalent bonds between the oxygen atom and the two hydrogen atoms.
Worksheet Wonders: A Hands-on Approach
Now, let’s delve into the exciting world of worksheets. These worksheets don’t need to be boring. They can be engaging and help you understand the concepts in a visual, interactive way:
Types of Worksheets:
- Bonding Types: Here, you could be tasked with identifying the types of bonds present in different molecules. This involves analyzing the chemical formulas and using your knowledge of electronegativity, which measures an atom’s ability to attract electrons. You’ll learn to recognize patterns such as the presence of metal and nonmetal elements in ionic compounds, or the sharing of electrons between similar nonmetal atoms in covalent bonds.
- Lewis Dot Structures: These diagrams, named after the American chemist Gilbert N. Lewis, provide a visual representation of the arrangement of valence electrons (electrons in the outermost shell) around an atom. By drawing these structures, you gain a deeper understanding of how atoms share or transfer electrons to form bonds. You’ll practice drawing Lewis dot structures, predicting molecular shapes, and identifying the types of chemical bonds.
- Bonding Properties: This type of worksheet focuses on the properties of ionic and covalent compounds. You’ll explore the differences in melting points, boiling points, solubility, and conductivity, relating these properties to the nature of the chemical bonds present.
Real-Life Applications: Seeing the Bonds in Action
The concepts of ionic and covalent bonding extend far beyond the pages of textbooks and worksheets. They are fundamental to understanding the world around us:
Life as We Know It:
- Water: The water molecule, H2O, is held together by two covalent bonds. These bonds account for the properties of water, essential for life, such as its high boiling point, surface tension, and ability to dissolve many substances. Without water, life as we know it wouldn’t exist.
- Proteins and DNA: The building blocks of life – proteins and DNA – are composed of long chains of molecules held together by covalent bonds. These bonds maintain the intricate structures of these molecules, crucial for their biological functions.
Science and Technology:
- Batteries: Ionic bonding plays a key role in batteries. The movement of ions through an electrolyte solution facilitates the flow of electricity. This principle is utilized in rechargeable batteries found in phones, laptops, and electric cars.
- Semiconductors: Covalent bonds are crucial in the field of semiconductors. The properties of silicon, a common semiconductor material, depend on the arrangement of its covalent bonds. These materials are fundamental to modern technology, enabling the creation of transistors, integrated circuits, and countless electronic devices.
Worksheet Chemical Bonding Ionic & Covalent
Conclusion: A Journey of Discovery
So there you have it! The world of chemical bonding, once seemingly complex, becomes clear and fascinating once you dive into it through worksheets and real-world examples. By understanding the fundamental principles of ionic and covalent bonding, you gain valuable insights into the fascinating world of atoms and molecules. Continue to explore chemical bonding concepts – you’ll be surprised at the connections you discover within the world around you! So, grab a worksheet, unleash your curiosity, and embark on your own exciting journey of discovery about the invisible forces that hold everything together!






