Remember that time you mixed baking soda and vinegar in a science experiment? The bubbling, fizzing reaction was a classic example of a chemical transformation happening right before your eyes. But did you know that this “fizzy” reaction represents an exothermic reaction, one that releases heat and energy into the surrounding environment? This is just one example of the fascinating world of chemical reactions, and understanding the difference between endothermic and exothermic reactions is crucial for anyone venturing into the world of chemistry.
Image: psiberg.com
In this comprehensive guide, we’ll delve deep into the concepts of endothermic and exothermic reactions, explore the key differences, and provide valuable tips for mastering this essential chemistry topic. We’ll even include a handy worksheet to solidify your understanding and prepare you for any upcoming exams or assignments. Let’s get started!
Defining the Basics: Endothermic and Exothermic Reactions
At their core, chemical reactions involve the breaking and forming of chemical bonds, leading to the creation of new substances. These reactions are accompanied by changes in energy, either releasing or absorbing energy. This energy exchange is what differentiates endothermic and exothermic reactions.
Endothermic reactions are reactions that require energy input to occur. They absorb energy from their surroundings, typically in the form of heat, leading to a decrease in the temperature of their surroundings. Think of it like absorbing warmth from a fireplace – the fireplace gets cooler as you absorb its heat. Imagine a cold pack that you use for injuries, these contain chemicals that undergo an endothermic reaction when mixed. Absorbing heat from your skin which makes you feel cooler.
Exothermic reactions, on the other hand, release energy into their surroundings, often resulting in an increase in temperature. An example of this is burning wood, which produces heat. To better understand, consider a bonfire; it releases heat and light into the surrounding environment, making you feel warmer. The combustion process in your car engine is another great example of an exothermic reaction.
Understanding the Energy Flow
To visualize the energy exchanges in endothermic and exothermic reactions, we can use energy diagrams or graphs. These diagrams illustrate the change in energy from the reactants to the products over the course of the reaction.
In an endothermic reaction, the products have higher energy levels than the reactants. This indicates that energy has been absorbed from the surroundings for the reaction to occur. The energy diagram will show an upward slope, representing the energy that is consumed. Think of climbing a hill; you need to put in energy (in this case, heat) to reach the higher ground (representing the products).
In an exothermic reaction, the products have lower energy levels than the reactants. This indicates that energy has been released into the surroundings. The energy diagram will show a downward slope, representing the energy that is being released. Think of rolling down a hill; the energy of the hill transforms into kinetic energy as you go down (representing the product forming).
Delving into the Details: Endothermic and Exothermic Reactions
Here are some crucial details that differentiate endothermic and exothermic reactions:
- Energy Change: Endothermic reactions consume energy (positive enthalpy change), while exothermic reactions release energy (negative enthalpy change).
- Temperature Change: Endothermic reactions absorb heat, causing a decrease in temperature, while exothermic reactions release heat, causing an increase in temperature.
- Enthalpy: Endothermic reactions have a positive enthalpy change, meaning that the system’s enthalpy increases. Conversely, exothermic reactions have a negative enthalpy change, indicating a decrease in the system’s enthalpy.
- Examples:
Endothermic: Photosynthesis (plants absorb energy from sunlight), melting ice cubes, evaporating water.
Exothermic: Burning fuel, explosion of dynamite, neutralization reaction of acid and base.
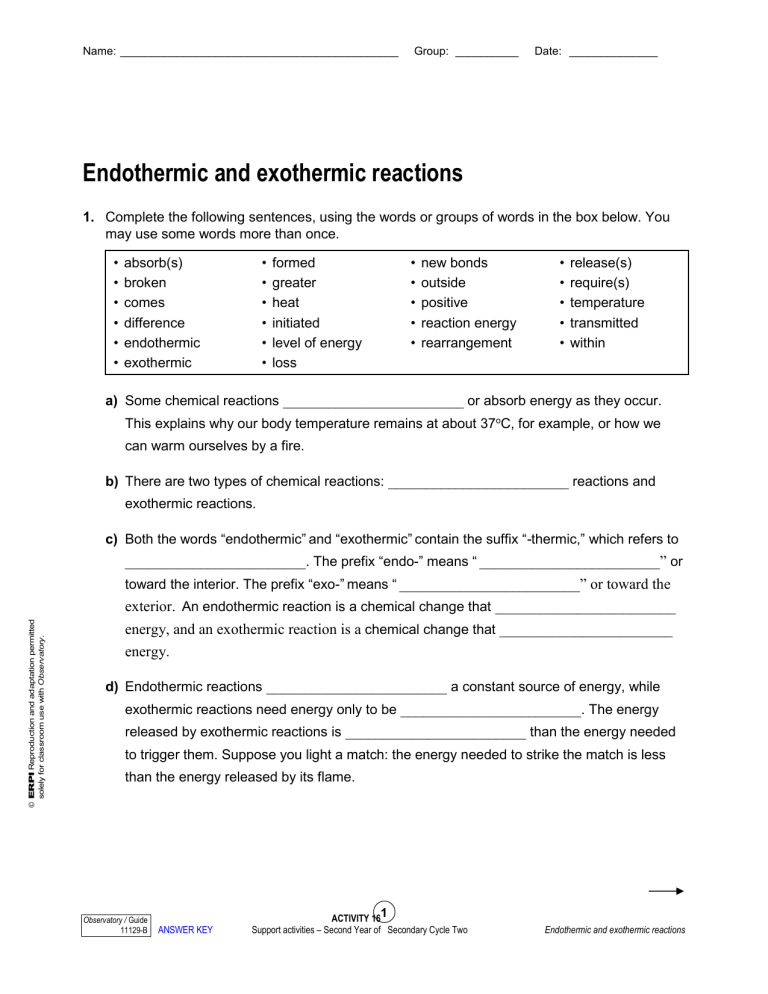
Image: www.vrogue.co
Applying Your Knowledge: Endothermic vs Exothermic Reactions Worksheet
Now let’s put your understanding to the test with a handy worksheet designed to reinforce your knowledge of endothermic and exothermic reactions. This worksheet will provide a comprehensive review of these concepts and their application.
Worksheet Activities:
- Identify Reactions: Analyze various chemical reactions and classify them as endothermic or exothermic based on their energy changes and temperature changes. For example, “Melting ice” is an example of a reaction that absorbs heat (endothermic).
- Energy Diagrams: Interpret energy diagrams for different reactions and determine if the reaction is endothermic or exothermic. For example, if the energy of the products is higher than the reactants, the reaction is endothermic.
- Real-life Examples: Identify real-world examples of endothermic and exothermic reactions encountered in everyday life. The combustion of gasoline in a car engine is an example of an exothermic reaction.
- Chemical Equations: Practice writing balanced chemical equations for endothermic and exothermic reactions.
Unlocking the Secrets: Tips and Expert Advice
Mastering endothermic and exothermic reactions involves more than just memorizing definitions. Here are a few expert tips to enhance your grasp of these concepts.
Visualize the Energy Flow: Instead of just relying on words, try to visualize the flow of energy in different chemical reactions. Imagine the energy being absorbed by the reactants or released by the products.
Connect with Real-life Applications: Look for real-life examples of endothermic and exothermic reactions around you. This will help you relate the abstract concepts to tangible experiences.
Practice Makes Perfect: Regularly practice solving problems and answering questions related to endothermic and exothermic reactions. Utilize worksheets, online exercises, and textbook examples to boost your understanding.
Frequently Asked Questions
Here are some common questions regarding endothermic and exothermic reactions:
Q: How can I tell if a reaction is endothermic or exothermic without knowing the enthalpy change?
A: You can determine whether a reaction is endothermic or exothermic by observing the temperature change of the surroundings. If the surroundings get colder, the reaction is endothermic. If the surroundings get warmer, the reaction is exothermic.
Q: What is the role of activation energy in endothermic and exothermic reactions?
A: Activation energy is the minimum amount of energy required for a reaction to occur, regardless of whether it is endothermic or exothermic. Endothermic reactions require energy input to overcome the activation energy, while exothermic reactions release energy, but still require activation energy to start the reaction.
Endothermic Reaction Vs Exothermic Reactions Worksheet
Summing It Up: Mastering the Energy of Reactions
Let’s recap – endothermic and exothermic reactions are fundamental concepts in chemistry that explain the energy changes associated with chemical transformations. This worksheet and expert tips should provide you with a solid foundation for understanding these reactions. We encourage you to keep exploring the fascinating world of chemistry and its everyday implications!
Are you interested in learning more about endothermic and exothermic reactions, or perhaps exploring other chemical reactions? Let us know in the comments below, and we’ll be happy to provide you with further resources and information!






